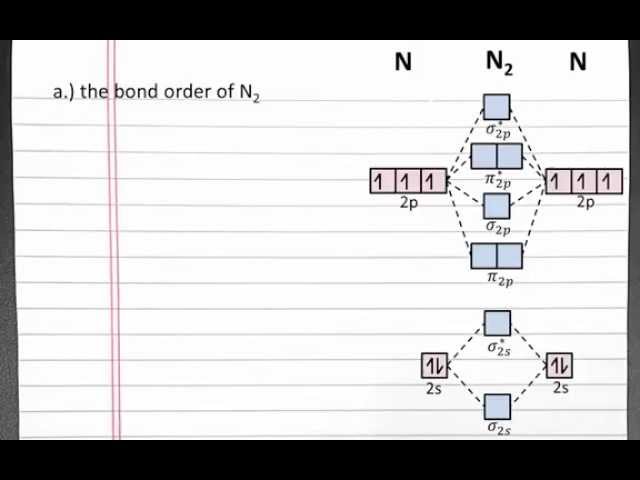
Bond order n2 is a measure of the strength of the chemical bond between two atoms. It is calculated using a formula that takes into account the number of electrons in the bonding and antibonding orbitals.
Bond order n2 is an important concept in chemistry because it can be used to predict the properties of molecules. For example, molecules with high bond orders tend to be more stable and less reactive than molecules with low bond orders.
The concept of bond order was first developed by Linus Pauling in the 1930s. Pauling’s work on bond order helped to lay the foundation for modern chemistry.
How to Calculate Bond Order n2
Bond order n2 is a measure of the strength of the chemical bond between two atoms. It is an important concept in chemistry because it can be used to predict the properties of molecules.
- Definition
- Formula
- Importance
- Applications
- Limitations
- Historical development
- Related concepts
- Further reading
These aspects of bond order n2 are all important to understand in order to be able to use it effectively. By understanding the definition, formula, importance, applications, limitations, historical development, related concepts, and further reading, you will be able to use bond order n2 to predict the properties of molecules and understand the nature of chemical bonds.
Definition
The definition of bond order n2 is critical to understanding how to calculate it. Bond order n2 is a measure of the strength of the chemical bond between two atoms, and it is calculated using a formula that takes into account the number of electrons in the bonding and antibonding orbitals. Without a clear understanding of the definition of bond order n2, it would be impossible to develop a formula for calculating it.
In practice, the definition of bond order n2 is used to predict the properties of molecules. For example, molecules with high bond orders tend to be more stable and less reactive than molecules with low bond orders. This understanding is essential for chemists who are trying to design new materials with specific properties.
The definition of bond order n2 is also important for understanding the nature of chemical bonding. Bond order n2 provides a quantitative measure of the strength of the bond between two atoms, which can help chemists to understand how atoms interact with each other.
Formula
The formula for calculating bond order n2 is a key aspect of understanding how to calculate bond order n2. The formula takes into account the number of electrons in the bonding and antibonding orbitals, and it can be used to predict the strength of the chemical bond between two atoms.
- Number of bonding electrons
The number of bonding electrons is the number of electrons that are in the bonding orbitals. These electrons are responsible for holding the atoms together.
- Number of antibonding electrons
The number of antibonding electrons is the number of electrons that are in the antibonding orbitals. These electrons are responsible for weakening the bond between the atoms.
- Bond order
Bond order is calculated by subtracting the number of antibonding electrons from the number of bonding electrons, and then dividing the result by 2.
The formula for calculating bond order n2 is a powerful tool that can be used to understand the nature of chemical bonds. By understanding the formula, you can predict the strength of the bond between two atoms, and you can also understand how the bond will be affected by changes in the number of bonding and antibonding electrons.
Importance
Understanding how to calculate bond order n2 is of great importance in chemistry. Bond order n2 is a measure of the strength of the chemical bond between two atoms, and it can be used to predict the properties of molecules. By understanding the importance of bond order n2, chemists can gain a deeper understanding of the nature of chemical bonding and how it affects the behavior of molecules.
- Predicting molecular properties
Bond order n2 can be used to predict a variety of molecular properties, including bond length, bond strength, and vibrational frequency. This information is essential for understanding the behavior of molecules and designing new materials with specific properties.
- Understanding chemical reactions
Bond order n2 can be used to understand the mechanisms of chemical reactions. By understanding the bond order of the reactants and products, chemists can predict the products of a reaction and the rate at which it will occur.
- Designing new materials
Bond order n2 can be used to design new materials with specific properties. For example, chemists can use bond order n2 to design materials that are stronger, more durable, or more resistant to heat.
- Understanding biological systems
Bond order n2 is essential for understanding the structure and function of biological systems. For example, bond order n2 can be used to understand the interactions between proteins and DNA, and how these interactions affect the function of cells.
In conclusion, understanding how to calculate bond order n2 is of great importance in chemistry. Bond order n2 is a powerful tool that can be used to predict the properties of molecules, understand the mechanisms of chemical reactions, design new materials, and understand biological systems.
Applications
The applications of bond order n2 are vast and varied, spanning many fields of science and engineering. Bond order n2 is a critical component of understanding the properties of molecules and materials, and it is used to design new materials with specific properties.
One important application of bond order n2 is in the field of materials science. Bond order n2 can be used to predict the strength and stability of materials, and it can be used to design new materials with improved properties. For example, bond order n2 has been used to design new materials for use in aerospace applications, where lightweight and durable materials are essential.
Another important application of bond order n2 is in the field of chemistry. Bond order n2 can be used to understand the mechanisms of chemical reactions, and it can be used to predict the products of a reaction. This information is essential for chemists who are trying to develop new drugs and other products.
In conclusion, bond order n2 is a powerful tool that has a wide range of applications in science and engineering. By understanding how to calculate bond order n2, scientists and engineers can gain a deeper understanding of the properties of molecules and materials, and they can design new materials with specific properties.
Limitations
It is important to be aware of the limitations of bond order n2 when using it to predict the properties of molecules. These limitations include the following:
- Assumes a simple molecular orbital picture
Bond order n2 assumes that the molecular orbitals involved in the bond are simple and non-degenerate. This is not always the case, and can lead to errors in the calculated bond order.
- Does not account for all types of bonding
Bond order n2 only takes into account covalent bonding. It does not account for other types of bonding, such as ionic bonding or metallic bonding.
- Can be difficult to apply to large molecules
Bond order n2 can be difficult to apply to large molecules, as it requires a detailed knowledge of the molecular orbitals involved.
Despite these limitations, bond order n2 is a useful tool for predicting the properties of molecules. It is important to be aware of the limitations of bond order n2 when using it, and to use it in conjunction with other methods to obtain a more complete picture of the bonding in a molecule.
Historical development
The historical development of how to calculate bond order n2 is a fascinating story that spans many decades. It is a story of scientific discovery and innovation, and it has led to a deeper understanding of the nature of chemical bonding.
- Early theories
The early theories of bond order were developed in the early 1900s. These theories were based on the idea that the strength of a bond is related to the number of electrons that are shared between the two atoms.
- Pauling’s work
Linus Pauling made significant contributions to the development of bond order theory in the 1930s. Pauling’s work showed that bond order can be used to predict the properties of molecules, such as bond length and bond strength.
- Modern developments
In the years since Pauling’s work, there have been many advances in the field of bond order theory. These advances have led to a better understanding of the nature of chemical bonding, and they have made it possible to calculate bond order more accurately.
- Current applications
Bond order theory is now used in a wide variety of applications, including the design of new materials and the prediction of the properties of molecules.
The historical development of how to calculate bond order n2 is a testament to the power of scientific inquiry. It is a story of how scientists have used their knowledge to gain a deeper understanding of the world around them.
Related concepts
Understanding the related concepts of bond order n2 is crucial for a comprehensive grasp of its calculation. These concepts encompass the fundamental components, applications, and implications of bond order n2.
- Molecular orbital theory
This theory provides the foundation for understanding the formation and properties of chemical bonds. It describes how atomic orbitals combine to form molecular orbitals, which determine the bond order and strength.
- Valence bond theory
This theory focuses on the overlap of atomic orbitals and the resulting electron pairs that form covalent bonds. It complements molecular orbital theory by providing an alternative perspective on bond formation and properties.
- Resonance
Resonance occurs when a molecule can be represented by multiple valid Lewis structures. This concept affects bond order calculations as it involves the delocalization of electrons and the averaging of bond orders over multiple resonance structures.
- Bond length
Bond length is inversely related to bond order. Stronger bonds, with higher bond orders, tend to have shorter bond lengths. This relationship provides valuable insights into the strength and nature of chemical bonds.
These related concepts collectively enhance our understanding of bond order n2 calculations. They provide a deeper foundation for interpreting and utilizing bond order as a predictive tool in chemistry.
Further reading
Further reading on how to calculate bond order n2 can provide valuable insights into its theoretical foundations, practical applications, and implications in various fields. By delving deeper into these resources, a comprehensive understanding of bond order n2 and its significance can be achieved.
- Theoretical background
Explore the fundamental principles and mathematical formulations behind the calculation of bond order n2, gaining a deeper understanding of its theoretical underpinnings.
- Applications in chemistry
Discover the diverse applications of bond order n2 in chemistry, including predicting molecular properties, understanding reaction mechanisms, and designing new materials.
- Computational methods
Learn about the computational techniques employed to calculate bond order n2, including molecular orbital theory and density functional theory, and their advantages and limitations.
- Case studies and examples
Examine real-life examples and case studies where bond order n2 calculations have been applied successfully, providing practical insights into its utility and impact.
Through further reading, a holistic understanding of how to calculate bond order n2 can be acquired, empowering researchers and practitioners to leverage this knowledge for advancements in chemistry and related disciplines.
FAQs on Bond Order n2 Calculation
This section addresses frequently asked questions and clarifies common misconceptions regarding the calculation of bond order n2.
Question 1: What is the significance of bond order n2 in chemistry?
Answer: Bond order n2 quantifies the strength and stability of chemical bonds, providing insights into molecular properties, reactivity, and overall behavior.
Question 2: How is bond order n2 calculated?
Answer: It is calculated using a formula that considers the number of electrons in bonding and antibonding molecular orbitals.
Question 3: What factors can affect bond order n2?
Answer: Factors such as the number of shared electrons, electronegativity differences, and resonance can influence the bond order.
Question 4: What are the limitations of bond order n2 calculations?
Answer: It assumes specific molecular orbital interactions and may not accurately reflect bond order in all cases, especially for complex molecules.
Question 5: How can bond order n2 be applied in real-world scenarios?
Answer: Bond order n2 finds applications in predicting material properties, designing new compounds, and understanding chemical reactions.
Question 6: What are some common pitfalls to avoid in bond order n2 calculations?
Answer: Incorrectly identifying molecular orbitals or neglecting resonance contributions can lead to inaccurate results.
These FAQs provide a concise overview of key aspects related to bond order n2 calculation. For further exploration, the next section delves into advanced concepts and applications of bond order n2.
Transition to the next section: In the following section, we will explore advanced topics in bond order n2 calculation, including its applications in molecular spectroscopy and computational chemistry.
Tips for Calculating Bond Order n2
This section provides practical tips to enhance your understanding and accuracy when calculating bond order n2. By following these tips, you can gain a deeper insight into the nature of chemical bonds.
Tip 1: Master Molecular Orbital Theory
Thoroughly understand the concepts of molecular orbital theory, including the formation, symmetry, and interactions of molecular orbitals.
Tip 2: Identify Bonding and Antibonding Orbitals
Correctly identify the bonding and antibonding molecular orbitals involved in the bond under consideration.
Tip 3: Count Electrons Carefully
Accurately count the number of electrons occupying the bonding and antibonding molecular orbitals.
Tip 4: Consider Resonance Structures
In cases of resonance, calculate the bond order by considering the average of bond orders over all contributing resonance structures.
Tip 5: Utilize Computational Tools
Leverage computational chemistry software or online tools to assist with complex bond order calculations.
Tip 6: Understand Limitations
Be aware of the limitations of bond order n2 calculations, such as the assumption of specific molecular orbital interactions.
Summary: These tips empower you to calculate bond order n2 with greater confidence and accuracy. By following these guidelines, you can gain valuable insights into bond strength, molecular stability, and chemical reactivity.
Transition: In the concluding section, we will explore the broader implications of bond order n2 calculations and their applications in various fields of chemistry and materials science.
Conclusion
This comprehensive exploration of how to calculate bond order n2 has provided deep insights into the nature of chemical bonds. By understanding the concepts of molecular orbital theory, identifying bonding and antibonding orbitals, and carefully counting electrons, we can accurately determine bond order n2, a crucial measure of bond strength and stability.
The key points highlighted in this article include the significance of bond order n2 in predicting molecular properties, its applications in various fields of chemistry and materials science, and the limitations and considerations involved in its calculation. These interconnected ideas empower chemists to design and understand molecules with tailored properties and explore the vast possibilities of chemical bonding.