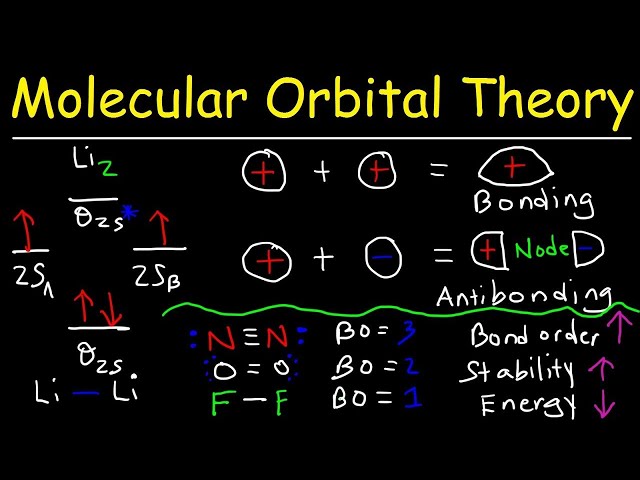
Molecular orbital theory is a fundamental quantum mechanical model used to describe the electronic structure of molecules. It provides a detailed and accurate understanding of chemical bonding, molecular geometry, and various properties of molecules. In this context, bond order is a crucial concept that represents the strength of a chemical bond between two atoms in a molecule. It helps predict the stability, reactivity, and overall characteristics of the molecule.
Bond order calculations are essential in various fields of chemistry, including inorganic chemistry, organic chemistry, and biochemistry. It plays a key role in understanding the electronic structure, bonding, and properties of molecules. Historically, the concept of bond order was first introduced by Linus Pauling, who proposed that the bond order between two atoms is equal to the number of electron pairs shared between them.
Understanding how to calculate bond order in molecular orbital theory is essential for chemists and researchers. In this article, we will explore the underlying principles, mathematical equations used, and practical applications involved in determining bond order. We will also discuss its significance and implications in comprehending chemical bonding and molecular properties.
How to Calculate Bond Order in Molecular Orbital Theory
Understanding how to calculate bond order in molecular orbital theory is paramount in chemistry. It encompasses various essential aspects that provide insights into the electronic structure and bonding of molecules.
- Determining the number of electrons in bonding and antibonding orbitals
- Identifying molecular symmetry and orbital overlap
- Applying Hckel’s rule to calculate bond order in conjugated systems
- Utilizing the concept of resonance to determine bond orders in resonance structures
- Employing MO theory to account for bond order in coordination complexes
- Describing the relationship between bond order and bond length
- Establishing the connection between bond order and bond strength
- Understanding the influence of bond order on molecular stability
- Exploring the role of bond order in predicting molecular properties
- Using bond order calculations to design and modify molecules with desired properties
These key aspects collectively contribute to a comprehensive understanding of the significance of bond order in molecular orbital theory. By exploring these aspects, we gain a deeper appreciation of the electronic structure of molecules, their bonding characteristics, and their overall properties.
Determining the Number of Electrons in Bonding and Antibonding Orbitals
Determining the number of electrons in bonding and antibonding orbitals is a crucial step in calculating bond order using molecular orbital theory. Bonding orbitals are formed by the constructive overlap of atomic orbitals, resulting in a decrease in energy compared to the original atomic orbitals. Antibonding orbitals, on the other hand, arise from the destructive overlap of atomic orbitals, leading to an increase in energy.
The number of electrons occupying bonding and antibonding orbitals directly influences the bond order. Bond order is defined as half the difference between the number of electrons in bonding and antibonding orbitals. A higher number of electrons in bonding orbitals and a lower number in antibonding orbitals result in a higher bond order, indicating a stronger bond. Conversely, a lower number of electrons in bonding orbitals and a higher number in antibonding orbitals lead to a lower bond order, indicating a weaker bond.
For instance, in the hydrogen molecule (H2), the molecular orbital formed from the overlap of the two atomic orbitals is a bonding orbital, and it is occupied by two electrons. Since there are no antibonding orbitals in H2, the bond order is 1, corresponding to a single bond. In contrast, in the oxygen molecule (O2), the molecular orbital formed from the overlap of the atomic orbitals is an antibonding orbital, and it is occupied by two electrons. The presence of the antibonding orbital and the occupation of electrons in it result in a bond order of 2, indicating a double bond.
Understanding how to determine the number of electrons in bonding and antibonding orbitals is essential for accurate bond order calculations. This understanding enables chemists to predict the strength and type of bonds in molecules, which is vital for comprehending their structure, reactivity, and properties.
Identifying molecular symmetry and orbital overlap
Identifying molecular symmetry and orbital overlap forms a cornerstone of calculating bond order using molecular orbital theory. The symmetry of a molecule determines the types of orbitals that can overlap and the extent of their overlap. Orbital overlap, in turn, directly influences the number of electrons that can occupy bonding and antibonding orbitals, which ultimately affects the bond order.
For instance, in a homonuclear diatomic molecule like H2, the molecular orbitals result from the overlap of atomic orbitals with the same symmetry. The atomic orbitals align in a way that maximizes overlap and results in the formation of a strongly bonding molecular orbital. In contrast, in a heteronuclear diatomic molecule like HCl, the atomic orbitals involved in bonding have different symmetries, leading to less overlap and a weaker bond.
The understanding of molecular symmetry and orbital overlap allows chemists to predict the bond order and, consequently, the strength and properties of chemical bonds. This knowledge is essential in various fields of chemistry, including inorganic chemistry, organic chemistry, and biochemistry. By analyzing molecular symmetry and orbital overlap, researchers can gain insights into the electronic structure, bonding, and reactivity of molecules, enabling them to design and modify molecules with desired properties.
In summary, identifying molecular symmetry and orbital overlap is a crucial step in calculating bond order using molecular orbital theory. It provides insights into the electronic structure of molecules, allowing chemists to predict and understand the strength and nature of chemical bonds. This understanding has far-reaching applications in chemistry and related fields, guiding the design of new materials, drugs, and technologies.
Applying Hckel’s Rule to Calculate Bond Order in Conjugated Systems
Within the framework of molecular orbital theory, applying Hckel’s rule offers a simplified method to calculate bond order in conjugated systems. It provides valuable insights into the electronic structure and bonding characteristics of these systems, which play a crucial role in determining their chemical properties and reactivity.
- Hckel Molecular Orbitals: Hckel’s rule focuses on -molecular orbitals formed by the overlap of p-orbitals in conjugated systems. These orbitals extend over multiple atoms, resulting in delocalization of -electrons.
- Cyclic Conjugation: Hckel’s rule is primarily applicable to cyclic conjugated systems, where the -orbitals form a closed loop. The number of -electrons and the symmetry of the determines the molecular orbital energies and the resulting bond orders.
- Bond Alternation: In certain conjugated systems, Hckel’s rule predicts alternating single and double bonds. This bond alternation pattern arises from the constructive and destructive interference of the -molecular orbitals, leading to varying bond lengths and strengths.
- Aromatic Systems: Hckel’s rule plays a central role in identifying aromatic systems, which are highly stable due to their resonance and delocalized -electron cloud. The 4n + 2 -electron rule, derived from Hckel’s theory, helps predict the aromaticity of cyclic conjugated systems.
By applying Hckel’s rule to calculate bond order in conjugated systems, chemists gain a deeper understanding of the electronic structure, bonding, and properties of these systems. This knowledge is essential in fields such as organic chemistry and materials science, where conjugated systems are prevalent and their properties are crucial for various applications.
Utilizing the concept of resonance to determine bond orders in resonance structures
Within the framework of molecular orbital theory, utilizing the concept of resonance to determine bond orders in resonance structures plays a crucial role in understanding the electronic structure and bonding characteristics of molecules. Resonance is a phenomenon that occurs when a molecule or ion can be represented by multiple Lewis structures, each with different arrangements of double and single bonds. These resonance structures contribute to the overall electronic structure and properties of the molecule.
To determine bond orders in resonance structures, chemists employ molecular orbital theory, which provides a more accurate and comprehensive description of the electronic structure compared to Lewis structures. Molecular orbital theory takes into account the wave-like behavior of electrons and the interactions between them, leading to a more detailed understanding of bond formation and properties.
In practice, determining bond orders in resonance structures using molecular orbital theory involves calculating the molecular orbitals and their corresponding energies. The bond order of each bond is then determined based on the number of electrons occupying the bonding and antibonding molecular orbitals. Resonance structures with lower energy and more stable molecular orbitals contribute more significantly to the overall electronic structure and bond orders of the molecule.
Utilizing the concept of resonance to determine bond orders in resonance structures finds applications in various fields of chemistry, including organic chemistry, inorganic chemistry, and biochemistry. It helps predict the stability, reactivity, and properties of molecules, guiding the design and synthesis of new materials and compounds with desired characteristics.
In summary, utilizing the concept of resonance to determine bond orders in resonance structures is an important aspect of molecular orbital theory, providing insights into the electronic structure and bonding of molecules. It enables chemists to make accurate predictions about molecular properties and reactivity, which are essential for advancing our understanding of chemical systems and designing new materials with tailored properties.
Employing MO theory to account for bond order in coordination complexes
In the realm of molecular orbital theory, employing MO theory to account for bond order in coordination complexes is a crucial aspect that deepens our understanding of the electronic structure and bonding characteristics of these complexes. Coordination complexes are formed when a metal center is bound to a group of ligands, and the nature of the metal-ligand bond is a fundamental aspect in determining the complex’s properties and reactivity.
MO theory provides a theoretical framework to describe the electronic structure of coordination complexes by considering the interactions between the metal d-orbitals and the ligand orbitals. Through the formation of molecular orbitals, electrons are delocalized over the entire complex, leading to the concept of bond order. Bond order in coordination complexes is a measure of the strength of the metal-ligand bond and is influenced by factors such as the number and type of ligands, the oxidation state of the metal, and the geometry of the complex.
Employing MO theory to calculate bond order in coordination complexes is essential for understanding their stability, reactivity, and magnetic properties. By determining the bond order, chemists can predict the strength of the metal-ligand bond, which in turn affects the complex’s overall stability. Furthermore, bond order provides insights into the electronic configuration of the metal center, influencing its reactivity and magnetic behavior.
Real-life examples of employing MO theory to account for bond order in coordination complexes are numerous. In organometallic chemistry, the bonding between transition metals and organic ligands can be described using MO theory, helping to explain the reactivity and catalytic properties of these complexes. In bioinorganic chemistry, understanding the bond order between metal ions and biological ligands is crucial for elucidating the structure and function of metalloproteins.
The practical applications of understanding bond order in coordination complexes extend to various fields, including catalysis, medicine, and materials science. In catalysis, the bond order between the metal center and the substrate influences the catalytic activity and selectivity of coordination complexes. In medicine, bond order is important for understanding the interactions between metal-based drugs and biological targets. In materials science, the design of coordination complexes with specific bond orders is crucial for developing materials with tailored properties, such as magnetism and conductivity.
In summary, employing MO theory to account for bond order in coordination complexes is a fundamental aspect of understanding the electronic structure, bonding, and properties of these important compounds. By calculating bond order, chemists can gain insights into the stability, reactivity, and magnetic behavior of coordination complexes, leading to practical applications in catalysis, medicine, and materials science.
Describing the relationship between bond order and bond length
Understanding the relationship between bond order and bond length is a fundamental aspect of molecular orbital theory. Bond order, which represents the strength of a chemical bond, is inversely related to bond length, meaning that stronger bonds tend to be shorter, while weaker bonds tend to be longer.
- Atomic Orbital Overlap: Bond order is determined by the extent of overlap between atomic orbitals. Stronger overlap leads to higher bond order and shorter bond lengths. For instance, in a double bond, the greater overlap between the p-orbitals results in a shorter bond length compared to a single bond.
- Bond Type: The type of bond (single, double, or triple) is directly related to bond order and bond length. Single bonds have the lowest bond order and longest bond length, while triple bonds have the highest bond order and shortest bond length.
- Resonance and Delocalization: In resonance structures, the delocalization of electrons can affect bond order and bond length. Delocalized electrons lead to a more even distribution of bonding, resulting in shorter and more uniform bond lengths.
- Hybridization: The hybridization of atomic orbitals also influences bond order and bond length. Hybrid orbitals with greater overlap, such as sp3 orbitals, lead to higher bond order and shorter bond lengths compared to pure atomic orbitals.
Describing the relationship between bond order and bond length provides valuable insights into molecular structure and bonding. It helps predict bond lengths and strengths, which are crucial for understanding chemical reactivity, molecular stability, and material properties. This understanding guides the design and synthesis of new materials with tailored properties and applications.
Establishing the connection between bond order and bond strength
Establishing the connection between bond order and bond strength is a fundamental aspect of molecular orbital theory. Bond order provides valuable insights into the strength and nature of chemical bonds, enabling chemists to predict and understand the behavior of molecules.
- Bond Energy: Bond order is directly related to bond energy. Strong bonds have high bond orders and high bond energies, while weak bonds have low bond orders and low bond energies.
- Bond Length: Bond order also correlates with bond length. Shorter bonds have higher bond orders, indicating stronger bonds, while longer bonds have lower bond orders, indicating weaker bonds.
- Reactivity: Bond order influences the reactivity of molecules. Molecules with higher bond orders are less reactive, while molecules with lower bond orders are more reactive.
- Physical Properties: Bond order affects various physical properties of molecules, such as melting point, boiling point, and electrical conductivity.
Establishing the connection between bond order and bond strength allows chemists to make predictions about the behavior of molecules and design materials with desired properties. By understanding the relationship between bond order and bond strength, researchers can tailor molecules for specific applications, ranging from pharmaceuticals to advanced materials.
Understanding the Influence of Bond Order on Molecular Stability
Understanding the influence of bond order on molecular stability forms a critical component of molecular orbital theory. Bond order provides valuable insights into the strength and nature of chemical bonds, which directly impact the stability and behavior of molecules.
By calculating bond order, chemists can predict the relative stability of different molecular structures. Higher bond orders indicate stronger bonds, which lead to more stable molecules. For instance, a molecule with multiple double or triple bonds is generally more stable than a molecule with only single bonds. Bond order also influences molecular geometry and reactivity. Molecules with higher bond orders tend to adopt more compact geometries and are less reactive due to the stronger bonds holding the atoms together.
The understanding of bond order’s influence on molecular stability has far-reaching applications in various fields. In materials science, it guides the design of materials with desired properties, such as strength, hardness, and thermal stability. In organic chemistry, it helps predict the reactivity and selectivity of organic molecules, enabling the development of more efficient and selective chemical reactions. Furthermore, in biochemistry, understanding bond order is essential for comprehending the structure and function of biomolecules, such as proteins and DNA.
In summary, understanding the influence of bond order on molecular stability is a crucial aspect of molecular orbital theory. By calculating bond order, chemists gain insights into the strength, stability, and behavior of molecules. This understanding has numerous practical applications, ranging from materials science to biochemistry, guiding the design and development of new materials and technologies.
Exploring the role of bond order in predicting molecular properties
Understanding bond order provides valuable insights into the behavior and properties of molecules. By exploring the role of bond order in predicting molecular properties, chemists can gain a deeper understanding of how bond strength and molecular structure influence various aspects of molecules.
- Bond Strength and Reactivity: Bond order is directly related to bond strength. Stronger bonds, indicated by higher bond orders, lead to less reactive molecules. This knowledge helps predict the reactivity of molecules and design chemical reactions accordingly.
- Molecular Geometry: Bond order influences molecular geometry. Molecules with higher bond orders tend to adopt more compact geometries to minimize bond lengths. This understanding helps predict molecular shapes and their impact on molecular properties.
- Spectroscopic Properties: Bond order affects the vibrational frequencies of bonds. Stronger bonds vibrate at higher frequencies. By analyzing spectroscopic data, chemists can determine bond orders and gain insights into molecular structure and dynamics.
- Magnetic Properties: Bond order is related to the magnetic properties of molecules. Molecules with unpaired electrons exhibit magnetic behavior. Understanding bond order helps predict the magnetic properties of molecules, which is crucial in fields such as materials science and spintronics.
Exploring the role of bond order in predicting molecular properties provides a powerful tool for understanding and manipulating molecules. By calculating bond order, chemists can make informed predictions about molecular behavior, design new materials with desired properties, and advance our knowledge of chemical systems.
Using bond order calculations to design and modify molecules with desired properties
Understanding how to calculate bond order in molecular orbital theory forms the foundation for using bond order calculations to design and modify molecules with desired properties. Bond order provides valuable insights into the strength and nature of chemical bonds, enabling chemists to predict and understand the behavior of molecules. By manipulating bond order, chemists can tailor molecules for specific applications, ranging from pharmaceuticals to advanced materials.
Real-life examples of using bond order calculations to design and modify molecules include the development of new drugs, the optimization of catalytic processes, and the creation of novel materials with tailored properties. In the pharmaceutical industry, bond order calculations help design drugs with specific binding affinities and biological activities. In catalysis, understanding bond order enables the design of catalysts with high activity and selectivity. In materials science, bond order calculations guide the development of materials with enhanced strength, durability, and electrical conductivity.
The practical applications of bond order calculations extend to various fields, including medicine, energy, and technology. By understanding how to calculate bond order in molecular orbital theory, chemists can harness this knowledge to address real-world challenges, such as developing new treatments for diseases, improving energy efficiency, and creating advanced materials for technological advancements.
FAQs on Calculating Bond Order in Molecular Orbital Theory
This section provides answers to frequently asked questions about calculating bond order using molecular orbital theory. These FAQs aim to clarify common misconceptions and provide additional insights into the concepts discussed in the main article.
Question 1: What is the significance of bond order in molecular orbital theory?
Answer: Bond order is a crucial concept in molecular orbital theory that indicates the strength and type of chemical bond between atoms. It helps predict molecular stability, reactivity, and various properties.
Question 2: How is bond order calculated in molecular orbital theory?
Answer: Bond order is calculated by determining the difference between the number of electrons in bonding and antibonding molecular orbitals and dividing the result by two.
Question 3: What factors affect bond order?
Answer: Bond order is influenced by factors such as the number of electrons involved in bonding, the overlap of atomic orbitals, and the symmetry of the molecular orbitals.
Question 4: How is bond order related to bond length and bond strength?
Answer: Bond order is inversely proportional to bond length, meaning shorter bonds have higher bond orders. Additionally, bond order is directly proportional to bond strength, indicating that stronger bonds have higher bond orders.
Question 5: What are the applications of bond order calculations?
Answer: Bond order calculations are used in various fields, including chemistry, physics, and materials science, to predict molecular properties, design new materials, and understand chemical reactions.
Question 6: What are some limitations of bond order calculations?
Answer: While bond order provides valuable insights, it is essential to note that it is an approximation and may not accurately reflect the complex interactions in all molecular systems.
These FAQs provide a concise overview of some key aspects related to calculating bond order in molecular orbital theory. A deeper understanding of these concepts is essential for harnessing the power of molecular orbital theory to explore and manipulate chemical systems.
In the next section, we will delve into advanced applications of bond order calculations, exploring how they are used to design and modify molecules with desired properties.
Tips for Calculating Bond Order in Molecular Orbital Theory
To enhance your understanding and application of bond order calculations in molecular orbital theory, consider these practical tips:
Tip 1: Master the basics of molecular orbital theory. A solid foundation in molecular orbital theory, including concepts like atomic orbitals, molecular orbitals, and electron configurations, is essential.
Tip 2: Utilize molecular orbital diagrams. Visualizing molecular orbital diagrams helps identify bonding and antibonding orbitals, crucial for bond order calculations.
Tip 3: Pay attention to symmetry. The symmetry of molecular orbitals influences their overlap and, consequently, the bond order.
Tip 4: Consider resonance structures. Resonance can affect bond order, especially in conjugated systems where electrons are delocalized.
Tip 5: Use computational tools. Computational chemistry software can assist in calculating bond orders accurately and efficiently.
Tip 6: Validate your results. Compare your calculated bond orders with experimental data or other theoretical methods to ensure their reliability.
By following these tips, you can confidently calculate bond orders using molecular orbital theory. This knowledge empowers you to explore and understand the electronic structure and bonding characteristics of molecules.
In the final section of this article, we will delve into advanced applications of bond order calculations, showcasing their significance in predicting molecular properties, designing new materials, and shaping the future of chemistry.
Conclusion
Throughout this article, we have explored the intricacies of calculating bond order using molecular orbital theory. Understanding bond order is paramount in chemistry, as it provides valuable insights into the electronic structure and bonding characteristics of molecules. Key ideas and findings include:
- Bond order is determined by the number of electrons in bonding and antibonding molecular orbitals.
- Factors such as atomic orbital overlap, molecular symmetry, and resonance influence bond order.
- Bond order is directly related to bond strength and inversely related to bond length.
These concepts are interconnected and provide a comprehensive understanding of bond order. By harnessing this knowledge, chemists can predict molecular properties, design new materials, and manipulate chemical reactions. The ability to calculate bond order empowers us to delve deeper into the world of molecular interactions, paving the way for scientific advancements and technological innovations.